No reductions seen in 28-day mortality or duration of hospital stay in COVID-19 with lopinavir-ritonavir
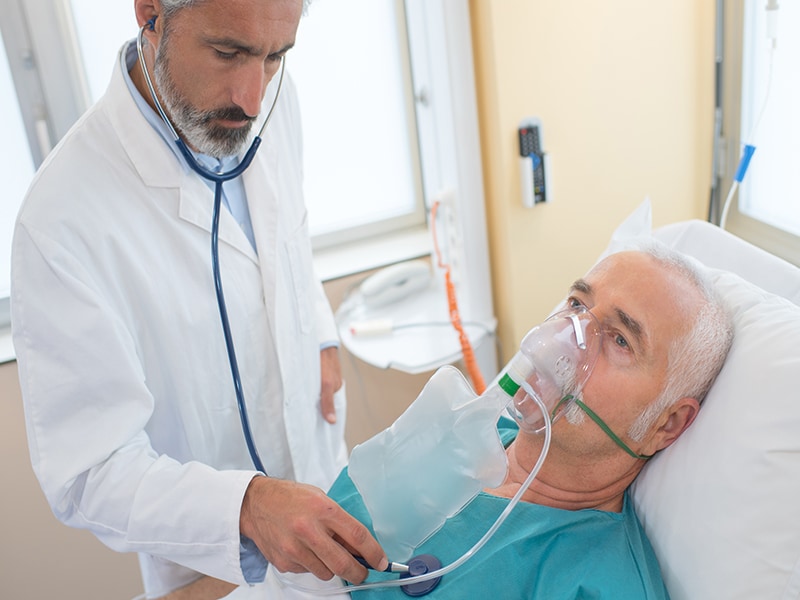
WEDNESDAY, Oct. 7, 2020 (HealthDay News) — Lopinavir-ritonavir is not associated with reductions in 28-day mortality or duration of hospital stay among patients admitted to the hospital with COVID-19, according to a study published online Oct. 5 in The Lancet.
Peter W. Horby, M.D., Ph.D., and colleagues on behalf of the RECOVERY Collaborative Group conducted a randomized open-label trial to compare treatments with usual care in patients admitted to the hospital with COVID-19. The trial was conducted at 176 hospitals in the United Kingdom. Eligible patients were randomly assigned to either usual standard of care alone (3,424 patients) or usual standard of care plus lopinavir-ritonavir (1,616 patients) for 10 days or until discharge.
The researchers found that 23 and 22 percent of patients allocated to lopinavir-ritonavir and usual care, respectively, died within 28 days (rate ratio, 1.03; 95 percent confidence interval, 0.91 to 1.17; P = 0.60). Across all prespecified subgroups, the results were consistent. There was no significant difference between the groups in the time until discharge from the hospital alive (median, 11 days in both groups) or the proportion discharged from the hospital alive within 28 days (rate ratio, 0.98; 95 percent confidence interval, 0.91 to 1.05; P = 0.53).
“The result from the RECOVERY trial is clear,” Horby said in a statement. “When combined with findings from an earlier, smaller trial and with the WHO interim results, this provides strong evidence that lopinavir-ritonavir is not an effective treatment for patients hospitalized with COVID-19.”
AbbVie contributed some supplies of lopinavir-ritonavir for use in this study; tocilizumab was provided free of charge by Roche.
Copyright © 2020 HealthDay. All rights reserved.